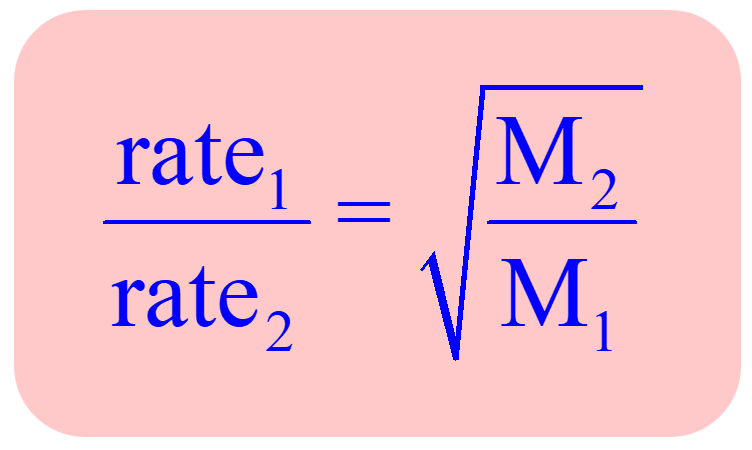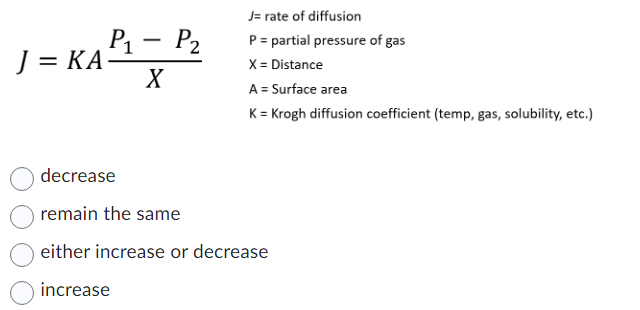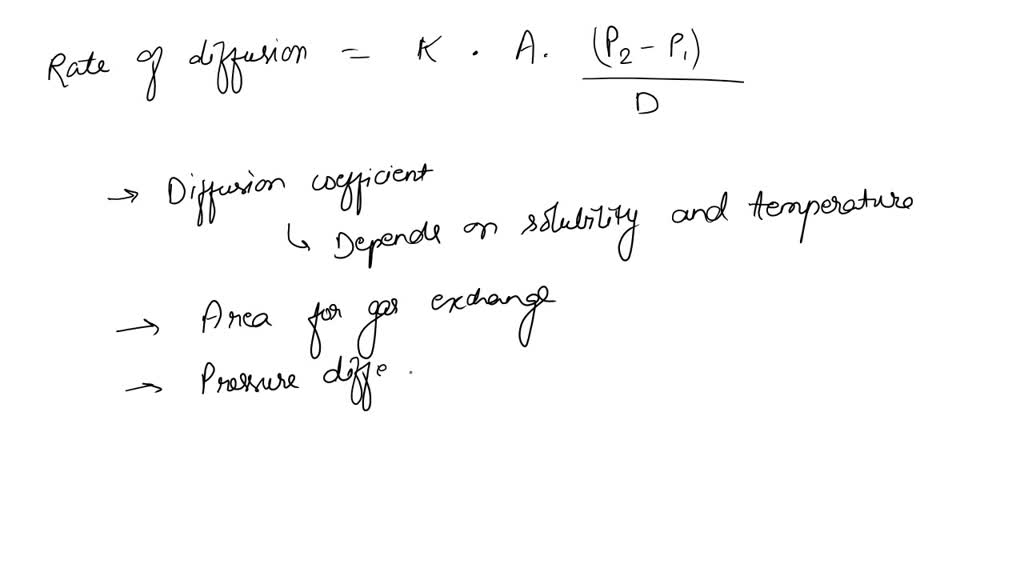
SOLVED: A) According to Fick's law of diffusion, what are four factors that affect the rate of diffusion? How do these factors affect the rate of diffusion? B) What are two specializations
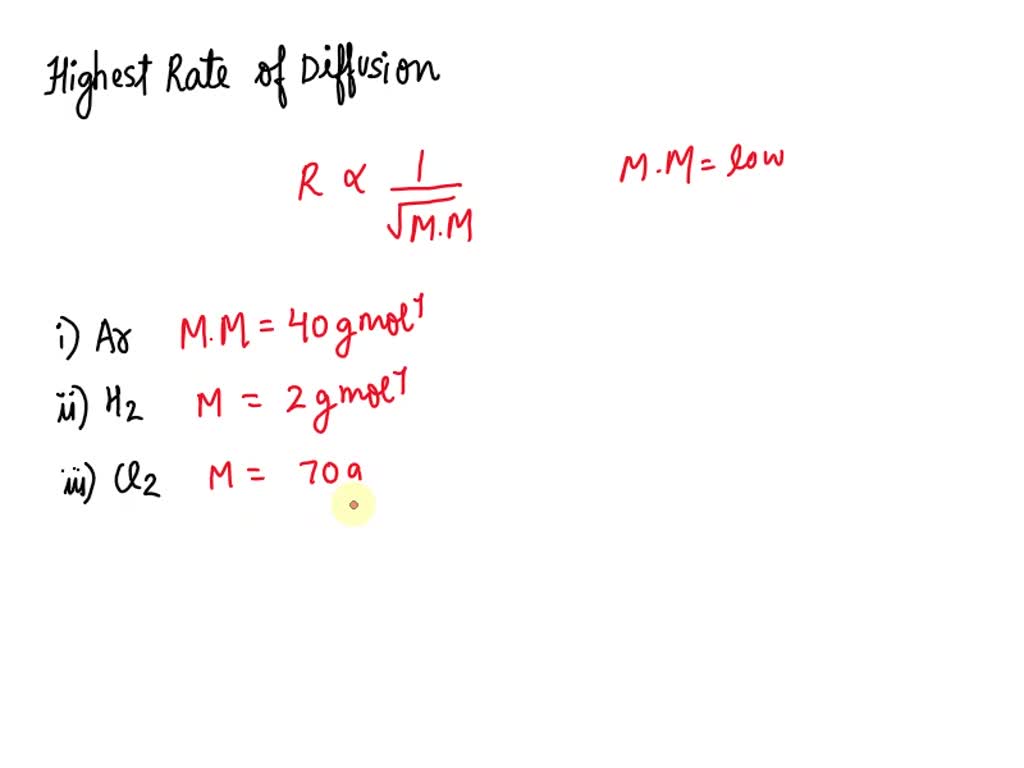
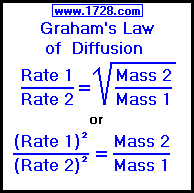
The relative rate of effusion of ch4 to so2 through the container containing ch4 and so2 in 3:2mass ratio
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora

The rate of diffusion of methane a given temperature is twice of a gas X . The molar mass of the gas X is?